▼A methylxan- what?
Being the most common in foods and beverages, calculating their concentrations in beverages would suffice in concluding if the levels of methylxanthine in our beverages are too high. But before doing any analysis, we must first understand their chemical properties.
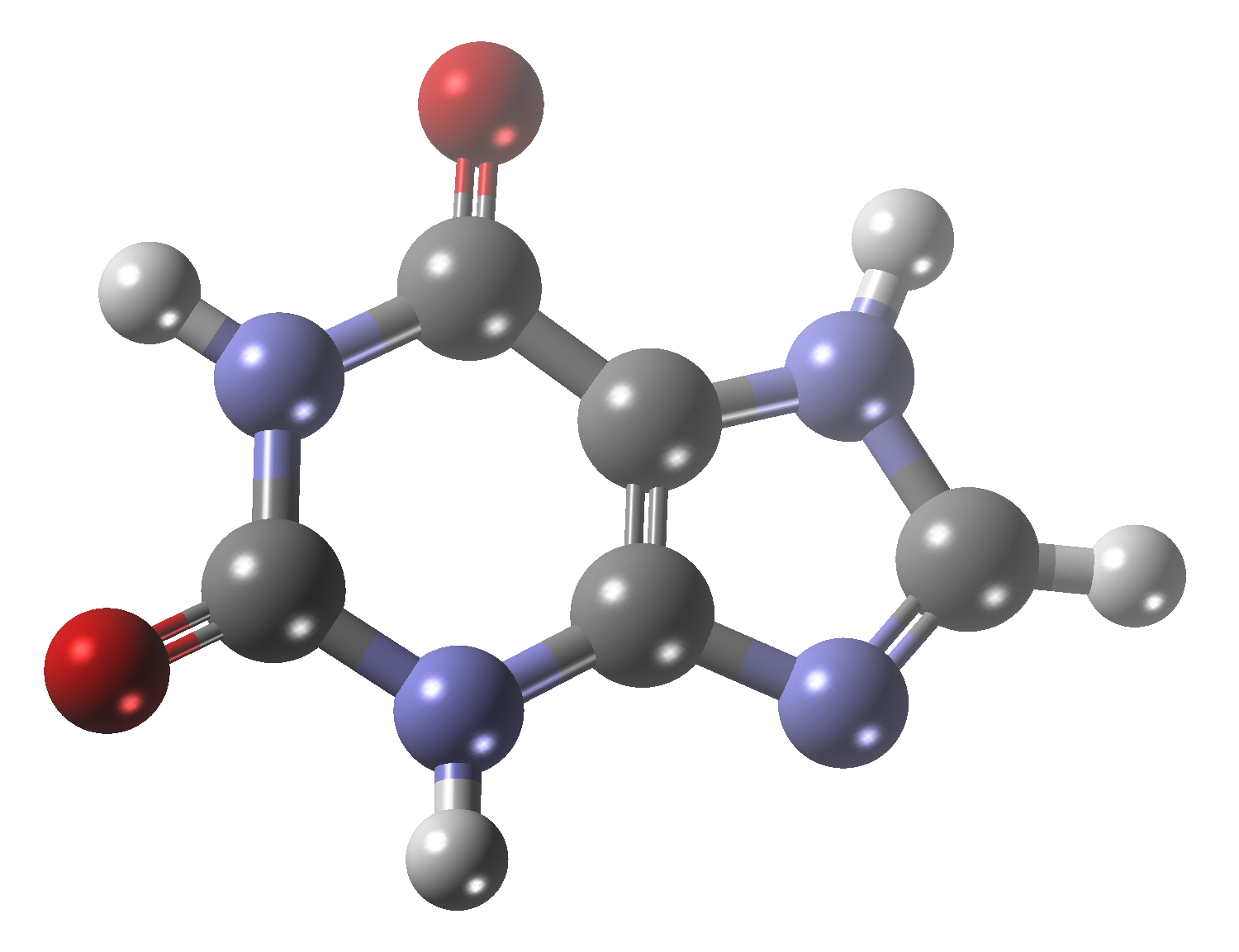
Theobromine
Materials
Stock Solutions of Caffeine, Theobromine and Theophylline
Acetic acid
Methanol (HPLC grade)
Pure Water
0.45µm membrane filter
Filter paper
Dark glass flasks
Varian liquid chromatograph Model 2510
Chemical Properties
Caffeine
Main functional group: Amine, Amide, Alkene, Purine
IUPAC name: 1,3,7-trimethyl-1H-purine-2,6(3H,7H)-dione
Molecular Formula: C8H10N4O2
Molecular Weight: 194.1906
Density: 1.23 g/cm³
Melting point: 238°C (sublimes)
Boiling point: 178°C (theoretical)
State at rtp: White colorless powder
Odour: None
Soluble in Water: 21600mg/L of H2O
Chromophores: Purine
Theobromine
Main functional group: Amine, Amide, Alkene, Purine
IUPAC name: 3,7-dimethyl-1H-purine-2,6-dione
Molecular Formula: C7H8N4O2
Molecular Weight: 180.164
Density: 1.50 g/cm³
Melting point: 290-295°C (sublimes)
Boiling point: 357°C (theoretical)
State at rtp: White colorless powder
Odour: None
Soluble in Water: 7360mg/L
of H2O
Chromophores: Purine
Theophylline
Main functional group: Amine, Amide, Alkene, Purine
IUPAC name: 1,3-dimethyl-7H-purine-2,6-dione
Molecular Formula: C7H8N4O2
Molecular Weight: 180.164
Density: 1.47 g/cm³
Melting point: 271-273°C (sublimes)
Boiling point: 454°C (theoretical)
State at rtp: White colorless powder
Odour: None
Soluble in Water: 330mg/L
of H2O
Chromophores: Purine
Methods
Based on the properties of these few methylxanthines, we are able to come up with a few methods of quantifying the amounts of methylxanthine in several sample beverages.
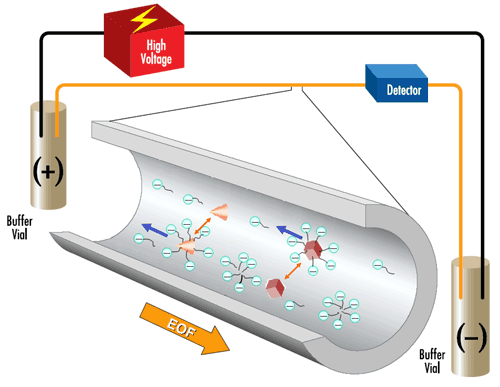
A) Micellar Electrokinetic Capillary Chromatography (MEKC)
Micellar Electrokinetic Capillary Chromatography (MEKC) is a chromatographic technique that is able to quantify the amount of methylxanthine in the sample. Samples are brewed in hot water and subsequently filtered to remove particulate matter before the chromatography
Pros: It is a very high resolution separation method.
Cons: Long separation time of >30 mins with poor resolution of late eluting peaks.
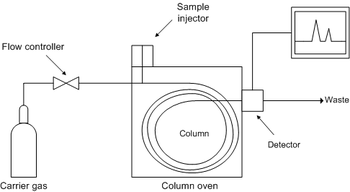
B) Gas Chromatography (GC)
Gas Chromatography (GC) is another viable method of quantifying the amount of methylxanthine in the sample. Samples are extracted using liquid-liquid extraction method and evaporated under a stream of nitrogen then reconstituted in 100 μL of methanol before analysis by the GC to ensure flash vaporisation. In a similar application, Helium was used as the carrier gas along with a Nitrogen-phosphorus detector (NPD)
Pros: Small amounts of sample is sufficient for analysis. Peak concentration is proportional to peak area, making it suitable for quantitative analysis.
Cons: Most of the methylxanthines are of low volatility hence requiring chemical derivatization. The liquid-liquid extraction of the samples is also a causative factor for more errors.
C) High-Performance Liquid Chromatography (HPLC)
High-Performance Liquid Chromatography (HPLC) is also an available option for the quantitation of the amount of methylxanthine in the sample. Samples are dissolved in hot water. Methanol-water-acetic acid should be used as a mobile phase with a suitable detector.
Pros: Fast analysis time without need for chemical derivatization.
Pros: Fast analysis time without need for chemical derivatization.
Cons: Irreversibly adsorbed compounds not detected, co-elution difficult to detect.
Selected analysis method: High-Perfomance Liquid Chromatography (HPLC)
As the samples require no pre-treatment(aside from dissolving in hot water) or chemical derivatization needed for analysis and the fact that the elution time is extremely short as compared to MEKC, HPLC would be the most preferred analysis method. Some researchers have also favoured HPLC as the method best suited for the analysis and have conducted the experiment as such.
Instruments and reagents used:
Chromatograph
Liquid chromatograph brand: Varian liquid chromatograph Model 2510
Injector type: Rheodyne injector with a 20 μL sample loop
Column length: 15cm
Column internal diameter: 4.0mm
Particle size: 5µm
Column stationary phase: Bondesil C18
Mobile phase: Methanol–Water–Acetic acid (20:75:5, v/v/v)
Mode: Isocratic elution
Flow rate of HPLC: 0.7ml/min
Injected volume for LC: 20 µl
Detector: Variable Wavelength Detector (Model: Varian UV–vis detector Model 2550)
Wavelength used for detection: 273nm
Instruments and reagents used:
Chromatograph
Liquid chromatograph brand: Varian liquid chromatograph Model 2510
Injector type: Rheodyne injector with a 20 μL sample loop
Column length: 15cm
Column internal diameter: 4.0mm
Particle size: 5µm
Column stationary phase: Bondesil C18
Mobile phase: Methanol–Water–Acetic acid (20:75:5, v/v/v)
Mode: Isocratic elution
Flow rate of HPLC: 0.7ml/min
Injected volume for LC: 20 µl
Detector: Variable Wavelength Detector (Model: Varian UV–vis detector Model 2550)
Wavelength used for detection: 273nm
Materials
Stock Solutions of Caffeine, Theobromine and Theophylline
Acetic acid
Methanol (HPLC grade)
Pure Water
0.45µm membrane filter
Filter paper
Dark glass flasks
Varian liquid chromatograph Model 2510
Reagent preparation:
Preparation of methylxanthine stock solutions
1. Stock solutions of caffeine, theobromine, and theophylline were prepared by dissolving 40 mg of each in 200 mL of methanol–water (50:50, v/v)
2. Filter through a 0.45-μm membrane filter
3. Store at 40°C in dark-glass flasks.
Preparation of methylxanthine calibration solutions
1. The standard solutions were prepared by dilution in the water of the methylxanthine mixture stock solutions within the concentration range of 0.25 to 60 μg/mL à At least 50x dilution
2. They were stored in the dark-glass flasks at 4°C. In these conditions they remained stable for 60 days.
Sample preparation
1. The soluble coffee was 150mL hot water per 1g of coffee
2. The tea was brewed with the tea-bag (2 g) and 150mL boiling water for 3 min
3. The cocoa consisted of 0.2 g chocolate per 20 mL water and was cleaned from grease by Soxhlet extraction with hexane
4. Coconut water was filtered
Process: High Performance Liquid Chromatography Tandem UV-Vis Spectrophotometry of Methylxanthine levels in beverage samples
Choice of Mobile Phase
Most of the methods described in the literature for methylxanthine analysis used mobile phases containing only methanol–water (pH~ 6.14) which allowed for caffeine quantitation but did not separate theophylline from theobromine. Methylxanthines can suffer protonation, which results in ionic species being stabilized (Figure 2). If the pH decreased below 4, the xanthines became protonated and the interaction with C18 reverse-phase columns increased. Using this knowledge, acetic acid was added to the mobile phase (apparent pH < 3) in order to increase the acidity, which resulted in good separation conditions. The HPLC determination was completed in approximately 12 min with the mobile phase methanol–water–acetic acid (20:75:5, v/v/v).
1. Stock solutions of caffeine, theobromine, and theophylline were prepared by dissolving 40 mg of each in 200 mL of methanol–water (50:50, v/v)
2. Filter through a 0.45-μm membrane filter
3. Store at 40°C in dark-glass flasks.
Preparation of methylxanthine calibration solutions
1. The standard solutions were prepared by dilution in the water of the methylxanthine mixture stock solutions within the concentration range of 0.25 to 60 μg/mL à At least 50x dilution
2. They were stored in the dark-glass flasks at 4°C. In these conditions they remained stable for 60 days.
Sample preparation
1. The soluble coffee was 150mL hot water per 1g of coffee
2. The tea was brewed with the tea-bag (2 g) and 150mL boiling water for 3 min
3. The cocoa consisted of 0.2 g chocolate per 20 mL water and was cleaned from grease by Soxhlet extraction with hexane
4. Coconut water was filtered
Risk assessment
Department: LSCTProcess: High Performance Liquid Chromatography Tandem UV-Vis Spectrophotometry of Methylxanthine levels in beverage samples
1. Hazard Identification
|
2. Risk Evaluation & Control
|
|||||||||||
SN
|
Task
|
Hazards
|
Possible Consequences
|
Existing Risk Control
(if any)
|
S
|
L
|
R
|
Additional / New Risk Control
|
S
|
L
|
R
|
Deadline
|
1
|
Elution with Methanol
|
Flammable and hazardous
|
Methanol may catch fire and causes skin burn
|
Place it far away from fire. eg. do not
place it near the spirit lamp
|
3
|
2
|
6
|
Handled under fume hood
|
3
|
1
|
3
|
26/01/2014
|
2
|
Dissolving of samples using hot water from heater
|
Physical thermal hazard on skin
|
May cause burns and scalding of skin
|
Wear heat resistant gloves when handling
|
2
|
4
|
8
|
-
|
-
|
-
|
-
|
26/01/2014
|
3
|
Handling of hexane when preparing chocolate
sample
|
Flammable and toxic to body
|
May cause respiratory tract irritation upon
inhalation
|
Handle under fume hood
|
3
|
1
|
3
|
Place it away from any naked flame available
|
3
|
1
|
3
|
26/01/2014
|
4
|
Detection using HPLC
|
Methanol might splash out if there are
contaminants present
|
May cause blindness if splashed on the eye
|
Wear lab goggles
|
3
|
4
|
12
|
Some labs have specialised areas for HPLC where
only people involved in the experiment are allowed access to it,
and they must be in complete protective gear.
|
3
|
4
|
12
|
26/01/2014
|
5
|
Clearing organic waste from the waste collector
of HPLC
|
Highly hazardous and flammable
|
May catch fire, cause skin burn, causes
irritation when inhaled and may cause blindness when splashed on eye
|
Wear chemical-resistant gloves and lab goggles
|
5
|
3
|
15
|
Handle in fume hood
|
5
|
3
|
15
|
26/01/2014
|
Results
Qualitative
▼Graph A and B
Graph A shows the standard methylxanthines and graph B shows the coffee sample with methanol-water-acetic acid through which by comparison of the location of the peak heights show that the method is able to qualitatively analyse the samples and identify the 3 different methylxanthines in the sample.
Quantitative
▼Table I
▼Table II
Table I above shows the calibration curves for the 3 methylxanthines that were obtained by analysis of the calibration solutions prepared.
Table II above shows the concentrations of the 3 methylxanthines in the samples that were tested by comparing peak heights of the readings from the analysis and comparing to the corresponding values in the plotted calibration curves.
Discussion
▼Figure 1
▼Figure 2
Choice of Mobile Phase
Most of the methods described in the literature for methylxanthine analysis used mobile phases containing only methanol–water (pH~ 6.14) which allowed for caffeine quantitation but did not separate theophylline from theobromine. Methylxanthines can suffer protonation, which results in ionic species being stabilized (Figure 2). If the pH decreased below 4, the xanthines became protonated and the interaction with C18 reverse-phase columns increased. Using this knowledge, acetic acid was added to the mobile phase (apparent pH < 3) in order to increase the acidity, which resulted in good separation conditions. The HPLC determination was completed in approximately 12 min with the mobile phase methanol–water–acetic acid (20:75:5, v/v/v).
▼Figure 5
Precision Testing
In order to ensure that the experiment is done accurately, a precision test was done by considering the stability of the standards and samples containing methylxanthines as it is one of the fundamental factors for the warranting of exact results. The study of the stability of the methylxanthine standards was conducted for 60 days. The standards were prepared in methanol and stored in dark glass flasks at 4°C. They showed good stability (Figure 5) and could be used safely for at least two months.
The determination of caffeine, theophylline and theobromine was also tested by using seven different aliquots which provided a relative standard deviation of 0.44%, 1.02%, and 0.64%, respectively, in peak-height variation, showing that the method is sufficiently precise.
Conclusion
Caffeine Levels
This report shows that common beverages consumed around the world all contained caffeine, theophylline and theobromine from the qualitative analysis albeit in different quantities. Through quantitative analysis, it is seen that the amount of methylxanthine present in beverages ranges from < 0.1pg/mL to 350 mg/mL for caffeine, < 0.1 pg/mL to 32 mg/mL for theobromine, and < 0.1 pg/mL to 47mg/mL for theophylline.
With recommended daily intake limits of methylxanthines for the average person at 400 mg, 39.05 mg and 900 mg for caffeine, theobromine and throphylline respectively, it is quite plausible to hit the threshold in which overdose symptoms like restlessness, cardiac arrhythmia, insomnia and dizziness can happen. In addition, recommended intake limits of methylxanthines can be even lower under other conditions, like in the case where a venti-size cup (~590ml) of our tested coffee A sample would be too much caffeine for pregnant women who have lowered limits of 200 mg a day! Hence, this calls for more precaution and more monitoring of the methylxanthines levels in our beverages we drink daily.
Method
The proposed method was shown to be appropriate for the separation and simultaneous quantitation of caffeine, theobromine, and theophylline in samples of food products, which presented high sensibility and quickness. The analysed samples needed no pretreatment or derivatization. They needed only filtration and if necessary a suitable dilution. Methanol is internationally one of the main solvents employed in liquid chromatography. Its substitution for the usual ethanol in mobile phases used in HPLC systems results in lower costs and toxicity. In this study, it shows the same effectiveness in the separation of methylxanthines. The application of this method can be applied in the control of formulations of medications because the three methylxanthines in this study are generally used as therapeutic agents, and, in food products, mainly because there exists suspicions that caffeine causes teratogenic activity.
The proposed method was shown to be appropriate for the separation and simultaneous quantitation of caffeine, theobromine, and theophylline in samples of food products, which presented high sensibility and quickness. The analysed samples needed no pretreatment or derivatization. They needed only filtration and if necessary a suitable dilution. Methanol is internationally one of the main solvents employed in liquid chromatography. Its substitution for the usual ethanol in mobile phases used in HPLC systems results in lower costs and toxicity. In this study, it shows the same effectiveness in the separation of methylxanthines. The application of this method can be applied in the control of formulations of medications because the three methylxanthines in this study are generally used as therapeutic agents, and, in food products, mainly because there exists suspicions that caffeine causes teratogenic activity.
References:
Bispo, M., Veloso, M., Pinheiro, H., Oliveira,
R., Reis, J. and Andrade, J. 2002. Simultaneous Determination of Caffeine,
Theobromine, and Theophylline by High-Performance Liquid Chromatography. 40
(1), pp. 45-48. Available from: doi: 10.1093/chromsci/40.1.45.
from: doi: 10.1093/chromsci/40.1.45.
Injac, R., Srdjenovic, B., Prijatelj, M.,
Boskovic, M., Karljikovic-Rajic, K. and Strukelj, B. 2008. Determination of
Caffeine and Associated Compounds in Food, Beverages, Natural Products,
Pharmaceuticals, and Cosmetics by Micellar Electrokinetic Capillary
Chromatography. 46 (2), pp. 137-143. Available from: doi: 10.1093/chromsci/46.2.137.
from: doi: 10.1093/chromsci/46.2.137.
Mccusker, R., Goldberger, B. and Cone, E. 2003.
Caffeine Content of Specialty Coffees. 27 (7), pp. 520-522. Available from: doi: 10.1093/jat/27.7.520.
from: doi: 10.1093/jat/27.7.520.
Scribd.com. 2014. Capillary
electrophoresis - eletroforese capilar. [online] Available at:
http://www.scribd.com/doc/4086007/13/Micellar-electrokinetic-capillary-chromatography
[Accessed: 26 Jan 2014].
wiseGEEK. 2014. How Much Caffeine is Consumed By the Average Person?. [online] Available at: http://www.wisegeek.com/how-much-caffeine-is-consumed-by-the-average-person.htm [Accessed: 26 Jan 2014].
Drugs.com. 2014. Theophylline Dosage - Drugs.com. [online] Available at: http://www.drugs.com/dosage/theophylline.html [Accessed: 29 Jan 2014].
at: http://www.drugs.com/dosage/theophylline.html [Accessed: 29 Jan 2014].
Roxby, P. 2011. How much coffee is safe?. [online] Available at: http://www.bbc.co.uk/news/health-15982904 [Accessed: 29 Jan 2014].
at: http://www.bbc.co.uk/news/health-15982904 [Accessed: 29 Jan 2014].
Toxnet.nlm.nih.gov. 2014. THEOBROMINE - National Library of Medicine HSDB Database . [online] Available at: http://toxnet.nlm.nih.gov/cgi-bin/sis/search/r?dbs+hsdb:@term+@DOCNO+7332 [Accessed: 29 Jan 2014].
. [online] Available at: http://toxnet.nlm.nih.gov/cgi-bin/sis/search/r?dbs+hsdb:@term+@DOCNO+7332 [Accessed: 29 Jan 2014].
wiseGEEK. 2014. How Much Caffeine is Consumed By the Average Person?. [online] Available at: http://www.wisegeek.com/how-much-caffeine-is-consumed-by-the-average-person.htm [Accessed: 26 Jan 2014].
Drugs.com. 2014. Theophylline Dosage - Drugs.com. [online] Available
 at: http://www.drugs.com/dosage/theophylline.html [Accessed: 29 Jan 2014].
at: http://www.drugs.com/dosage/theophylline.html [Accessed: 29 Jan 2014].Roxby, P. 2011. How much coffee is safe?. [online] Available
 at: http://www.bbc.co.uk/news/health-15982904 [Accessed: 29 Jan 2014].
at: http://www.bbc.co.uk/news/health-15982904 [Accessed: 29 Jan 2014].Toxnet.nlm.nih.gov. 2014. THEOBROMINE - National Library of Medicine HSDB Database
 . [online] Available at: http://toxnet.nlm.nih.gov/cgi-bin/sis/search/r?dbs+hsdb:@term+@DOCNO+7332 [Accessed: 29 Jan 2014].
. [online] Available at: http://toxnet.nlm.nih.gov/cgi-bin/sis/search/r?dbs+hsdb:@term+@DOCNO+7332 [Accessed: 29 Jan 2014].